Abstract
Introduction. Anti-CD19 chimeric antigen receptor T cells (CART19, CTL019) have led to impressive clinical responses in relapsing or refractory (r/r) B-cell acute lymphoblastic leukemia (B-ALL). With the likely imminent approval of CTL019 for patients with B-ALL, increasing numbers of patients will be exposed to this therapy. Last year we reported a rare case of a single patient who relapsed with CD19-negative, CAR19-expressing leukemia, likely due to inadvertent transduction of a leukemic cell with the CAR19 lentivirus during CTL019 manufacturing (Lacey SF, ASH, 2016 #281). The high expression of the CAR in leukemic cells and its absence from normal tissues make it an ideal engineered tumor target. We therefore developed anti-CAR19 CAR T cells with the goal of specifically targeting CAR19+ B-ALL.
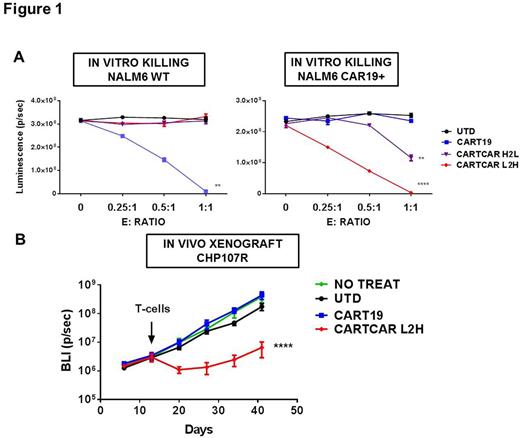
Methods. We designed two anti-CAR19 CAR using an anti-CAR19 idiotype scFv (clone 136.20.1, original clone kind gift of Dr. Laurence Cooper) testing two light chain orientations for the scFv: light to heavy (L2H) and heavy to light (H2L). We cloned the constructs into our standard backbone containing the CD8 hinge and trans-membrane domains, 4-1BB costimulatory and CD3z signaling domain. The constructs were packaged into a lentiviral vector and used to transduce normal donor T cells. As target cells, we used ex-vivo expanded primary leukemic blasts from the CAR19-expressing relapsed patient (CHP-107), as well as a luciferase+ B-ALL cell line (NALM6) transduced with the full-length CAR19 used in our clinical studies. Of note, the expression of CAR19 in CD19+ NALM6 cells led to apparent loss of CD19 by flow cytometry. We tested CART function in vitro with luciferase-based killing assays and in vivo in human xenograft models using NOD-SCID gamma chain deficient (NSG) mice.
Results. Both L2H and H2L anti-CAR19 CAR were efficiently expressed on T cells as detected by flow cytometry. In vitro CART-CAR19 efficiently killed CAR19-expressing B-ALL (NALM6) (L2H orientation: p< 0.0001; H2L: p=0.0063) but not wild-type (WT) NALM6 (p=NS) (Figure 1 A). In vivo engraftment studies showed that expression of CAR19 did not impact the growth rate of NALM6. We then tested our lead anti-CAR19 CART (L2H) in vivo. NSG mice were engrafted with either CAR19+ NALM6 (CD19-) or luciferase-positive relapsed CHP107 leukemia blasts (CAR19+, CD19-). At day 7 or 14 mice were randomized to receive no treatment, control T cells (UTD), CART19 or CART-CAR19 L2H. In both xenograft models CART19 and UTD cells failed to control disease progression, whereas CART-CAR19 showed significant leukemia control (Figure 1 B).
Conclusions. The acquisition of CAR expression by leukemic cells during CART manufacturing is a rare event that generates a tumor-specific antigen, thereby presenting an opportunity for specific targeting without off-target toxicity. In addition, we hypothesize that anti-CAR19 CART could be used as an "antidote" to CAR19 T cells for those patients who have been in stable deep remission for several years, in order to deplete their CAR T cells and alleviate B cell aplasia.
Ruella: Novartis: Patents & Royalties, Research Funding. Lacey: Novartis: Research Funding; Genentech: Honoraria. Melenhorst: Novartis: Research Funding. June: Celldex: Honoraria, Membership on an entity's Board of Directors or advisory committees; Immune Design: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; WIRB/Copernicus Group: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Patents & Royalties, Research Funding; Tmunity Therapeutics: Equity Ownership, Research Funding. Gill: Novartis: Patents & Royalties, Research Funding; CARMA Therapeutics: Consultancy, Equity Ownership, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal